

- #COMPENSATION FLOWJO HOW TO#
- #COMPENSATION FLOWJO MANUAL#
Compensation is correctly set when the median of the negative population is equal to the median of the positive population in the spillover channel. Compensation can be difficult if there is a wide range of fluorescence intensities in negative populations, which in turn can compromise sensitivity. This will become more of an issue as more fluorochromes are used. Do not forget to check the compensation in all channels.Īlthough most of the overlap will occur in the range on the higher wavelength side of the peak emission, due to the shape of the spectral emission curve, there may also be some bleed through/overlap to the opposite end, below the peak emission. As a general rule, compensate with the fluorochromes from the far-red end of the spectrum (higher wavelength), step-wise down to those fluorochromes at the lower end of the spectrum (lower wavelength). The use of the same batch for tandem conjugates is recommended. Tandem dyes (eg PE-Cy5) are more problematic due to variation in chemical conjugation. If a marker of interest is rare or possibly absent in the control cells, a different antibody directed against a more common marker can be used as long as it carries the same fluorochrome. If this is not possible, consider the use of compensation beads.Īlternatively, different cells for your compensation controls can be used as long as they express the markers of interest. Ideally, the positive and negative populations in the control samples will be the same type of cell. The autofluorescence of the positive population, before staining, should be the same as the negative control. The positive should be at least as bright as anything that will be encountered in the experiment and should form at least 10% of the population. These controls should be solely used to set compensation. Compensation controls are required for each fluorochrome and should contain both a positive and a negative population. Some fluorochrome combinations should be avoided if possible (eg APC and PE-Cy5), given the high degree of emission overlap. Dead cells, clumps and debris should be excluded from further analysis. Adjust forward scatter and side scatter so that the cell population is clearly delineated. Set voltages for fluorescence channels using an unstained sample. Ensure that the cytometer is performing within specifications using standard beads. 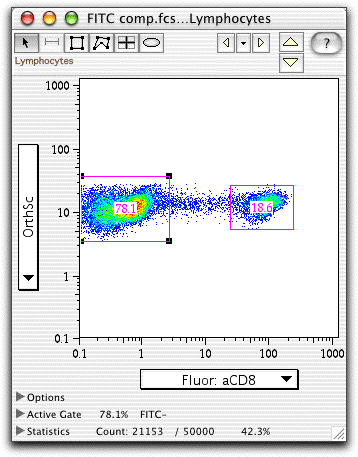
We always recommend reviewing the flow cytometer manufacturer's instructions for detailed compensation guidelines. However, the following guidelines should be suitable in most cases. The procedure for setting correct fluorescence compensation is essentially the same on any cytometer but there are differences between the various available instruments, which makes it difficult to provide a "one size fits all" protocol. (PE-Cy5 + PE overlap) - (PE overlap) = accurate PE-Cy5 results General procedure Adjust the compensation settings until no PE signal is seen in the PE-Cy5 channel (see the procedure below).Observe the signal in both PE and PE-Cy5 channels. Run a sample stained only with a PE-labeled antibody.Excitation and emission spectral profiles This will be seen as "false positive" signals in the PE-Cy5 channel and fluorescence compensation is needed to correct for this overlap. In the example below, following excitation with 488 nm light, PE emission is largely detected in the detector specific for PE but the emission tail lies within the range of the bandpass filter used for detection of PE-Cy5. This ensures that the fluorescence detected in a particular detector derives from the fluorochrome that is being measured. To correct for this spectral overlap, a process of fluorescence compensation is used. However, when emission spectra overlap, fluorescence from more than one fluorochrome may be detected. Within a flow cytometer, the appropriate ranges of excitation and emission wavelengths are selected by bandpass filters. The excitation spectrum is a range of light wavelengths that add energy to a fluorochrome, causing it to emit light in another range of wavelengths, the emission spectrum. What is compensation?Īll fluorochromes have excitation and emission spectra.
#COMPENSATION FLOWJO MANUAL#
Please refer to the manufacturer's instructions and software manual for a more detailed compensation procedure for your instrument.
#COMPENSATION FLOWJO HOW TO#
This article describes why compensation is required for flow cytometry and how to apply it.






 0 kommentar(er)
0 kommentar(er)
